Assessing the exposure to the UV filter DHHB in urine samples from the German Environmental Specimen Bank (2000–2021): Evaluating the impact of a potential impurity of di-n-hexyl phthalate in DHHB
Scherer, Max; Scherer, Gerhard; Riedel, Kirsten; Koch, Holger M.; Wrobel, Sonja A.; Murawski, Aline; Lemke, Nora; Weber, Till; Pluym, Nikola; Kolossa-Gehring, Marike
Int J Hyg Environ Health 266 (2025), online: 20 March 2025
Human biomonitoring (HBM) has become a crucial tool for assessing exposure to emerging chemicals. We analyzed 250 24-h urine samples from the German Environmental Specimen Bank (ESB), collected between 2000 and 2021, for exposure to diethylamino hydroxybenzoyl hexyl benzoate (DHHB), a UV filter increasingly used in sunscreens. Three major metabolites were examined: 2-(4-diethylamino)-, 2-(4-ethylamino)-, and 2-(4-amino)-2-hydroxybenzoyl)benzoic acid (DHB, EHB, AHB), with detection rates of 18◦%, 13◦%, and 87◦%, respectively. While EHB and DHB were specific to DHHB, AHB suggested other exposure sources, making it unreliable for assessing DHHB exposure. DHB and EHB were first detected in 2012, with increased detection rates thereafter. The median daily intake of 37 ng/kg bw/d was much lower than the derived no-effect level of 2900 mg/kg bw/d, indicating low risk from DHHB exposure. However, since the analyzed ESB samples were collected in winter, they likely reflect exposure from other products and the environment rather than sunscreen-related exposure.
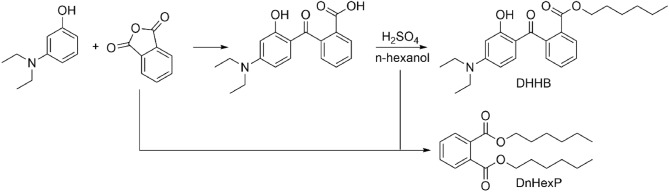
Recently, concerns have emerged regarding the DHHB impurity di-n-hexylphthalate (DnHexP), a reproductive toxicant not authorized in the EU. Retrospective analysis of oral DHHB dosing experiments indeed revealed impurity related dose-dependent excretion of DnHexP metabolites (MnHexP, oxidized 5-OH-MnHexP, and 5-oxo-MnHexP). Due to uncertainties in dose allocation, only a rough excretion fraction of 45◦% for MnHexP was derived. Our findings suggest that the DHHB impurity DnHexP may contribute to DnHexP exposure in sunscreen users applying products with contaminated DHHB. Given DnHexP’s toxicity, this warrants re-assessment of DHHB’s safety in cosmetics and enhanced surveillance of both DHHB and DnHexP in HBM studies.
https://doi.org/10.1016/j.ijheh.2025.114565